Isooctanol: Chemistry, History, Uses, and the Road Ahead
Historical Development
Isooctanol doesn’t draw much attention outside the labs and factories, yet it carries a long history in the industrial world. Chemists in the early 20th century started tinkering with branched-chain alcohols during the search for additives for fuels and plasticizers. Isooctanol took off as the motor and plastics industries boomed. Manufacturers saw a molecule with reliability under heat and chemical attack and put it to work everywhere from plastic films to hydraulic fluid. Over decades, refining processes have become more efficient and less wasteful. Today, most plants focus on selective synthesis to limit byproducts and speed up production, reflecting both regulatory pressure and higher demand for clean, consistent chemicals.
Product Overview
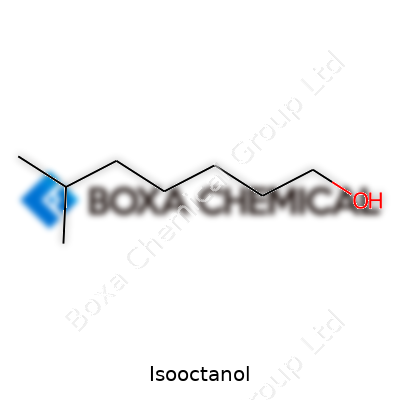
See a barrel with “Isooctanol” on the side and you’re looking at an alcohol with eight carbons and a chunky, branched structure. Factories turn this liquid into plasticizers, surfactants, lubricants, and fuel additives. Compared to straight-chain alcohols, isooctanol brings greater stability in heat and sunlight, making it a favorite for long-lasting polymers and special industrial fluids. Market reports show rising demand, especially in Asia-Pacific, driven by the growth in flexible PVC and detergent manufacturing.
Physical & Chemical Properties
Pure isooctanol flows as a colorless liquid at room temperature, carries a characteristic odor, and feels oily to the touch. Its boiling point sits near 179°C, a bit above water, which lets it survive many industrial processes without vaporizing away. The molecular weight lands at 130.23 g/mol. Isooctanol stands out for its limited water solubility, but it dissolves in most organics. With a flash point roughly 63°C, careful handling matters in the plant. Its branched structure—2-ethylhexanol the technical name—gives unique reactivity, so it resists oxidation and keeps polymers flexible even after months outdoors.
Technical Specifications & Labeling
Producers bottle isooctanol in drums with clear hazard labeling. Certificates of analysis must tick off purity, generally above 99%, with low levels for aldehydes, water, and acids. Regulations dictate that labels warn about flammability, inhalation risk, and environmental hazards, reflecting the chemical’s profile. Transport rules fall under UN Number 1993 “Flammable Liquids, N.O.S.” across most countries. On invoices, you’ll often see alternative names cropping up—this mix of labeling and paperwork comes from overlapping regulatory and industry norms worldwide.
Preparation Method
Petrochemical routes dominate isooctanol synthesis. In the lab, chemists rely on aldol condensation—usually starting with n-butyraldehyde and an alkali catalyst, followed by hydrogenation. Over time the industry slashed waste, improving catalyst lifespans and recycling more solvents. Modern sites design their reactors to handle scaling up without clogging or tripping safety systems. Small pilots tested continuous-flow reactors, and today’s biggest plants run day and night with fewer shutdowns thanks to real-time sensors in every pipe.
Chemical Reactions & Modifications
Isooctanol serves as both workhorse and workbench in the lab. It esterifies easily with acids, spawning a range of plasticizers used in everything from vinyl flooring to gaskets. It stands up to moderate bases and acts as a reducing agent in specific catalytic systems. Under some conditions, you can oxidize it to yield 2-ethylhexanoic acid, which finds use in lubricants and oil additives. Reactivity curves shift depending on solvents and temperature, but the predictable behavior lets chemical engineers design reliable batch processes. Demand for better plasticizers and specialty surfactants keeps the modification game going strong.
Synonyms & Product Names
Chemists and traders alike rattle off a tangle of names. “2-Ethylhexanol” ranks as the most recognized, but you’ll catch “Isooctyl alcohol,” “Octanol-2,” or even “EH alcohol” on paperwork. CAS number 104-76-7 keeps things unambiguous. Regional branding lands different names on the same drum, but regulatory and safety sheets keep the identity clear. Synonym confusion seldom trips up the pros, but mix-ups sometimes crop up with less seasoned buyers poking around for “octanol,” which covers a broader class of compounds.
Safety & Operational Standards
Workers in chemical plants handle isooctanol behind gloves, splash goggles, and proper ventilation. Flammable vapor can build up, especially in hot processes, so engineers rely on explosion-proof fittings and detailed risk assessments. Inhaled fumes irritate the upper airways, and spills call for fast containment; the compound floats on water but clings in soil, so every plant runs spill drills and posts clear instructions for cleanups. Europe’s REACH directive and OSHA standards in the United States set tight thresholds for worker exposure and flammability controls. Systematic hazard evaluations keep mishaps down, but training and vigilance make the difference.
Application Area
Isooctanol shows up everywhere flexible polymers get made—PVC pipes, flooring, cable insulation, and more. Plasticizer producers react it with phthalic anhydride for durable, bendable materials in construction and electronics. Detergent makers value its surfactant properties, spinning up specialty cleaning agents that work in hard water. Lubricant and oil additive formulators lean on its ability to reduce viscosity and resist chemical breakdown. Carbon-copy applications pop up worldwide because the molecule offers a balance of low cost and robust performance. Consumer-facing use is rare, as nearly all isooctanol leaves the factory inside another product.
Research & Development
R&D teams hunt for new catalysts to push yields higher and waste lower. Biocatalytic routes get explored, promising greener, renewable feedstocks, but petrochemical synthesis holds the cost advantage. Scientists work on direct conversion of biomass, aligning with carbon-neutral goals, and develop alternative plasticizers for stricter environmental standards. Testing investigates blends with biosurfactants to ramp up performance while reducing toxicity, reflecting market pressure for safer, more sustainable products. Analytical chemists look at impurity profiling and residuals in end-user products, driving refinement in manufacturing processes. Collaboration between academia and industry picks up as environmental scrutiny intensifies.
Toxicity Research
Toxicologists have put isooctanol through its paces, tracking short- and long-term exposure outcomes in animals and cell cultures. Animal studies generally show low acute toxicity, but high doses can damage kidneys and liver over time. Skin absorption is possible and calls for care in bulk handling. Chronic exposure studies fuel debates over permissible workplace concentrations, but data so far suggest limited risk at recommended limits. Like many chemicals in widespread use, regulators keep updating guidance as new research comes in, and downstream producers monitor for residuals in consumer products to safeguard public health. Environmental studies focus on aquatic toxicity, as runoff and spills can stress fragile water systems.
Future Prospects
The world’s appetite for softer plastics and specialty chemicals keeps demand for isooctanol climbing year by year. Companies invest in smarter reactors and renewable feedstocks, chasing lower emissions and tighter process control. New chemical building blocks, tougher environmental rules, and shifting consumer preferences push the industry to innovate. Biotechnological advances promise routes from plant matter instead of crude oil, and regulatory trends steer research into safer additives and greener solvents. In my view, real progress will come from tighter industry collaboration, transparent reporting, and ongoing investments in worker and environmental safety. Future products—whether plasticizers, surfactants, or fuels—stand to benefit if developers listen to both science and the shifting voices of regulation and public concern.
What Is Isooctanol?
Isooctanol sounds like something most folks would never run across, but it plays a role in things many people use every day. Chemically speaking, it belongs to a group called fatty alcohols. It shows up as a clear, oily liquid that comes from refining crude oil or from plant-based materials. The unique structure gives it qualities that industries count on, especially when crafting products that need a bit more performance or stability.
Building Better Fuels
Years ago, I discovered that isooctanol helps in producing plasticizers and surfactants, but I didn’t realize it has a hand in our fuel, too. Isooctanol turns up during the creation of isooctane — a vital component in gasoline. Adding isooctane boosts a fuel’s octane rating. That rating matters because it stops engines from knocking or rattling. Without additives like isooctanol, lots more engines would run rough or wear out faster. So, isooctanol actually helps drivers avoid unexpected trips to the shop, saving both time and money.
Everyday Products in the Home
Walk around the house, and products with a smoother texture or more lasting scent probably owe something to isooctanol. This alcohol’s special properties mean it blends well in cleaners, paints, and coatings. Surfactants, made using isooctanol, help water and oil mix, so stains and dirt lift away. In my own cleaning routine, the difference between a streaky surface and a spotless shine often comes down to hidden ingredients like this. Not many consumers think about what makes paint glide on evenly or why plastic toys stay flexible, but isooctanol stands behind many improvements.
The Challenge: Safety and Environmental Impact
One area that often gets overlooked involves the safety and environmental cost of massive isooctanol production. Workers in chemical plants deal with the risks of skin and eye irritation, and, in rare cases, breathing problems. Factory leaks or spills might cause problems for local water supplies and wildlife. In my early days working near an industrial park, I saw the worry firsthand whenever a smell drifted over nearby neighborhoods. It reminded me that safer handling and better containment need steady attention, not just for the workers but the whole ecosystem.
As society pushes for greener processes, some companies shift to using isooctanol made from renewable sources like palm oil, rather than just petroleum. This comes with its own problems, like deforestation linked to palm plantations. So, it pushes the industry to look for cleaner alternatives and monitor supply chains more closely. As a consumer, I’ve started to pay closer attention to sourcing, since even small choices can add up across millions of bottles or gallons of fuel.
Moving Forward with Responsibility
Isooctanol isn’t just a chemical off in a lab somewhere — it’s a workhorse hiding in plain sight, behind softer plastics and better fuels. Safer practices, cleaner sourcing, and customer transparency can all play a part in keeping its benefits without trading away health or environmental quality. As science keeps rolling forward, staying informed and asking for improvements keeps companies honest and helps communities stay safe.
Getting to Know Isooctanol
Isooctanol stands out as a valuable chemical used across many industries, particularly in the manufacture of plasticizers, surfactants, and lubricants. The chemical formula for isooctanol is C8H18O. Looking closer, this formula points to a molecule with eight carbon atoms, eighteen hydrogen atoms, and a single oxygen atom. This structure forms the backbone of the compound’s unique properties, such as its medium-chain alcohol nature and its slightly branched shape.
Importance Beyond the Lab
Seeing isooctanol just as a set of letters and numbers doesn’t show its actual impact. Many people, myself included, have encountered isooctanol without realizing because it shows up in common products. I remember working on a project involving PVC plastics and noting the soft, workable texture. That texture relies on plasticizers, many of which use isooctanol. The chemical’s structure makes it perfect for that purpose. It keeps plastics flexible and long-lasting, which matters in anything from tubing to flooring.
Isooctanol also finds a place in some lubricants and detergents. The molecule’s branching helps it mix well in formulations, balancing oil- and water-friendly qualities. Companies value this ability since it helps improve the function and performance of coatings and cleaners. In surfactants, for instance, the balance created by this formula translates straight to better cleaning results or moisture resistance. Everyday items like cleaners and conditioners often benefit from these kinds of molecular adjustments.
Industry Trends and Health Considerations
Across many chemical processes, safety and environmental impact keep growing in importance. Having worked in chemical handling, I learned to appreciate substances that deliver performance while minimizing risks. Isooctanol’s moderate volatility and relatively low toxicity compared to short-chain alcohols make it a reasonable choice. Worker safety and consumer protection depend on knowing the details: with a clear chemical formula, teams know exactly how to store, transport, and handle it.
Still, nothing in chemistry comes risk-free. In production environments, vapors or spills from liquid isooctanol may irritate the skin, eyes, or airways. Routine use of gloves, eye protection, and proper ventilation turns theory into practice. It matters for both workers and anyone down the line exposed to products containing industrial alcohols.
Environmental and Supply Chain Context
Raw materials for isooctanol often originate from petrochemical processes. Given today’s drive for sustainability and responsible sourcing, questions come up about how we make and use such compounds. Recently, efforts to shift toward greener manufacturing have picked up steam. Some producers look to bio-based alcohols that match isooctanol’s formula but start from renewable sources like plant oils instead of fossil fuels.
That pursuit brings challenges. It takes investment, new expertise, and public support to move supply chains toward cleaner options. Having the right chemical structure isn’t enough – companies, regulators, and consumers play key roles in driving better practices. Pushing for transparency about both the source and processing of compounds ensures the industry keeps improving health and safety outcomes, as well as sustainability.
Looking Ahead
Understanding a chemical’s formula isn’t just a technical matter. The combination of C8H18O in isooctanol reflects decades of progress in making household and industrial products more effective and affordable. But formula knowledge also helps buyers, workers, and scientists push manufacturers to address safety, reduce pollution, and innovate with newer, cleaner sources. Staying informed lets people make better choices — whether in the lab, on the factory floor, or at the store.
The Basics of Isooctanol
Isooctanol shows up in a lot more places than most people realize. You’ll notice it on the ingredient lists of cleaning agents, lubricants, and even flavor or fragrance additives. Straight from my days working in a chemical storeroom, I remember the strong scent when handling drums of the stuff. Isooctanol looks and pours like many other colorless organic liquids, which doesn’t make its safety much easier to judge on sight.
Toxicity and Immediate Hazards
It’s tempting to lump all industrial chemicals into the “dangerous” bucket; stories about chemical spills and workplace accidents get plenty of headlines. Toxicity needs some perspective, though. Isooctanol, or 2-ethylhexanol, won’t kill you from a whiff or two. The acute oral toxicity for rats is over 2,000 mg/kg, according to European safety assessments, which lands it outside most high-toxicity classifications. Touching small amounts with bare hands usually causes little concern, but it can cause skin and eye irritation. Anyone who’s wiped up a few splashes knows it gives a stinging sensation and can dry out your skin.
Breathing in vapors could make you dizzy or cause headaches, a risk that rises when working in enclosed spaces. I’ve seen people underestimate ventilation in labs and workshops, so opening windows and wearing simple gloves remain no-nonsense solutions. Long-term health issues haven’t cropped up for most professional handlers, but safety data sheets still recommend minimizing skin contact and avoiding inhalation.
Environmental Impact
Isooctanol doesn’t hang around long in soil or water. Microbes break it down in days to weeks. The bigger concern comes from letting it reach rivers and lakes, as high concentrations will harm aquatic life. Official testing points to moderate toxicity for small creatures living in streams and ponds. Good industrial practice means keeping it where it belongs—inside closed systems or safely contained during transport and use.
On a personal level, seeing chemical barrels rinse out at a plant site taught me just how quickly spills can turn into headaches for waste management teams. Any team worth its salt stores used isooctanol and contaminated residues in sealed drums until a licensed disposal company takes it away.
Human Health Evidence and Regulations
Consumers rarely bump into pure isooctanol. In factories, workers need to pay attention—eye protection, gloves, and basic ventilation go a long way. The European Chemicals Agency notes possible irritation but states that regular occupational exposure, under controlled conditions, leads to minimal health effects. The Environmental Protection Agency doesn’t classify it as a likely human carcinogen and long-term studies haven’t linked it to birth defects or serious disease.
From experience, clear labeling and communication in work environments help maintain safety. Product labels and training make a difference. When I gave safety talks, showing a basic chart of effects and personal stories actually got more attention than just waving a binder of rules.
Potential Solutions and Smarter Handling
Engineering controls cut down on risk—closed dispensing systems, fume hoods, and routine leak checks cut worries to a bare minimum. Training new hires (not just handing them a manual) gets everyone watching out for each other. Plenty of accidents happen not from toxic effects, but from slipping or splashing on the job. Good housekeeping—keeping a clean workspace and wiping up spills right away—protects people and equipment.
Focusing on responsible storage, up-to-date training, and prompt cleanup keeps isooctanol out of the headlines. Most problems happen when habits get sloppy. Sometimes it’s not about what a chemical can do, but what people fail to do. That’s the lesson my years in the industry keep drilling into me.
Why Proper Storage Matters
Anyone who has worked in a plant, warehouse, or lab knows chemical storage shapes safety and quality. Isooctanol deserves special attention, not just because it’s a clear, oily liquid, but because it brings real risks if handled carelessly. Take it from those of us who have felt the skin tingle after a spill or caught a whiff in the air. This alcohol can catch fire, irritate skin and eyes, and cause other health issues if it seeps into the wrong space.
Keeping It Contained
The right container makes all the difference. Metal drums with tight-sealing lids keep vapor from escaping and keep outside moisture away. Too much warmth or sunlight raises the chance of vapor and ultimately trouble—most plants place these drums in a dim, well-ventilated area far from ignition sources. Forklifts, static discharge, a dropped wrench, or something as simple as a spark from faulty wiring could lead to flames in the blink of an eye.
Over time, I’ve learned to look for the basics—a space with enough room for air, shelving that doesn’t wobble, and floors without cracks or buildup. It’s easy to rush, but stacking drums high, blocking barrels with clutter, or leaving leaks unchecked leads to mess and danger down the line.
Ventilation Isn’t Just a Detail
Anybody who’s worked a long shift knows the nagging headache from poor air. Isooctanol vapor settles fast in closed areas. Good ventilation flushes fumes and ensures anyone working nearby can breathe easy and think straight. Many facilities use extraction fans, and those should run any time barrels get moved, opened, or sampled. You’ll also catch more spills or leaks when air flows well. Keeping the nose sharp and eyes open saves headaches—sometimes literally.
Labeling and Security Make Work Safer
Nobody likes confusion in the storeroom. Clear labels cut through mistakes, especially on days when shifts change or new folks cover old ground. Emergency contact numbers, hazard icons, and handling instructions belong on every drum. Never underestimate the difference a big “Flammable” sign makes during an inspection or emergency.
Doors that lock, controlled key access, and a sign-in log help track who moves or samples chemicals. In the worst cases, misuse or theft puts more than property at risk—it puts lives and neighborhoods in danger.
Fire and Spill Controls Are Non-Negotiable
You learn quick in this job that speed beats panic. Keep fire extinguishers in arm’s reach, check them monthly, and make sure everyone has had a practice run on how to use them. Sand, absorbents, and well-lit spill kits cut cleanup time and keep the building from smelling like last week’s accident. I’ve seen folks toss rags in a bin and walk away, but that only spreads the chemical and sets up the next mistake.
Routine Checks and a Little Training Go a Long Way
Even the best plan falls apart without habit. Regular walk-throughs catch missing labels, slips in safety, and old containers swelling under the heat. Quick team meetings keep minds sharp, share stories that stick, and pass on the tricks old hands use to spot trouble before it grows. Good training isn’t just a binder on a shelf—it’s the stories and routines that remind every worker why the rules matter.
Taking isooctanol seriously means thinking ahead, talking straight, and always choosing the safer move, even when no one's watching. That’s how you avoid the kind of accident that ends work early and keeps folks up at night.
The Role of Isooctanol in Plasticizer Production
Walking down any supermarket aisle, plastic wraps, tubing, and wiring catch the eye without much thought about the chemicals lurking beneath the surface. Isooctanol, an alcohol with eight carbon atoms, shapes many of these products. The plastic manufacturing sector depends heavily on isooctanol for making plasticizers—compounds that keep plastics flexible and resistant to cracking. Manufacturers mix it into polyvinyl chloride (PVC), so you get water pipes that last longer and medical tubing that doesn't become brittle. While regular folks might not know exactly how these compounds come together, there's real value in recognizing that behind every flexible wire or hospital IV line sits a chemical that supports durability and safety.
Isooctanol in Lubricant Additives
There’s a good reason engine oils and gear greases run smoothly inside cars and industrial machines. Additives made from isooctanol change how lubricants behave under heavy stress. It’s not just about keeping engines from grinding to a halt, but also from overheating and wearing down too soon. Isooctanol offers stable chemical properties that resist high temperatures found in modern engines. Drivers rarely think about the bloodstream of their vehicles, but mechanics and plant engineers know how small improvements at the molecular level can mean longer life for machinery and lower maintenance bills for everyone.
Surfactants and Household Products
Every household relies on detergents, cleaning sprays, and personal care items. Isooctanol forms the building block for surfactants, which help mix oil and water, remove stubborn stains, and keep shampoo bottles sudsy to the last drop. Big cleaning brands look for consistent performance, and isooctanol delivers. Surfactant producers turn to this alcohol to reach the sweet spot between cleaning power and gentle skin contact. When washing the kitchen floor or shampooing pets, families benefit from safer and more efficient formulas made possible through straightforward chemistry.
The Flavors, Fragrances, and Agricultural Connection
Isooctanol sometimes pops up in food flavoring and fragrances, bringing subtle notes that enhance everyday experiences. In smaller doses, chemists use it to adjust the taste and aroma in packaged foods and scented products. Agricultural businesses tap into isooctanol for making pesticides and herbicides work better. By binding active ingredients together or enhancing how solutions spread across crops, isooctanol minimizes waste and helps farmers raise better yields with fewer resources. Agriculture faces mounting pressures from climate change and resource limitations; chemicals like isooctanol make a measurable difference by maximizing effectiveness in the field.
Safety and the Road Ahead
Working with industrial chemicals always brings its share of challenges and concerns. Isooctanol, like many chemical intermediates, can irritate the skin or eyes if mishandled. Factory workers and lab staff rely on proper protective equipment and clear protocols. Companies producing or using isooctanol focus on following international safety standards. Regulatory agencies demand transparency and traceability to manage environmental impacts. As global demand grows, industry leaders should keep reviewing greener production routes, exploring bio-based feedstocks, and minimizing hazardous emissions. Everyone connected to the supply chain—from chemical engineers to customers—can push for alternatives that respect personal health and keep communities safe.