Carbon Tetrachloride: A Hard Look at a Once-Popular Chemical
Historical Development
Back in the 19th century, chemists learned how to produce carbon tetrachloride on an industrial scale, aiming for a solvent that could tackle tough jobs no other chemical seemed to handle quite as effectively. Frederick Guthrie set the stage by making it from chloroform and chlorine. Demand ramped up through the twentieth century, with industries using it in refrigeration, fire extinguishers, cleaning, and even as a grain fumigant. Folks found it in household products and the dry cleaning industry for decades, but safety overlooked health risks in those years. With more research pointing to strong toxic effects, regulators clamped down, and production shifted toward more controlled, industrial applications.
Product Overview
Today, carbon tetrachloride serves as a solvent or process agent in specialized areas like petrochemicals and laboratory use. Pure samples appear as a clear, sweet-smelling liquid — easy enough to store in glass or metal because it does not corrode these materials under normal conditions. Many companies now market it for research purposes with strict labeling, reflecting current safety expectations and regulatory frameworks. Consumer-grade products no longer carry carbon tetrachloride, a result of accumulated evidence around its dangers and movement toward safer, greener alternatives.
Physical & Chemical Properties
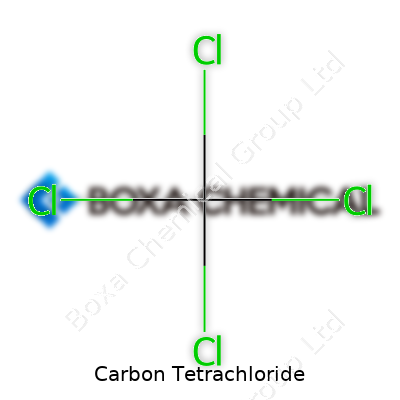
Carbon tetrachloride is a dense, colorless liquid, heavier than water and almost insoluble in it. Its boiling point hovers around 77°C, and it evaporates fast when exposed to air. Sweet or chloroform-like odors can make it seem less dangerous than it truly is. Chemically stable in the absence of strong bases or metals, it resists burning, which led folks to stick it in fire extinguishers long ago. Under ultraviolet light and in contact with metals, it breaks down and releases phosgene, a toxic gas, so storage requires careful distancing from heat and reactivity. It belongs to the halomethane group, with the formula CCl₄, and acts as a non-polar solvent.
Technical Specifications & Labeling
Producers batch carbon tetrachloride according to set purity standards, often exceeding 99% for lab-grade reagents. Quality checks focus on minimizing impurities, especially any water or hydrolyzed products, since those can skew research or manufacturing outcomes. Safety data sheets come bundled with each shipment and typically reference global hazard codes: GHS labels warn of acute toxicity and environmental harm. Regulations require rigid transport and packaging, with detailed hazard communication, signal words, and specific pictograms that leave no doubts about risks. Synthetic chemists pay attention to batch numbers and expiration dates, reducing the margin for error in sensitive applications or research projects.
Preparation Method
Industrially, most modern processes rely on chlorination of methane or methyl chloride. Factories push chlorine gas through these feedstocks at high temperatures, triggering a free-radical reaction. This stepwise method begins with mono-chlorination before forming CCl₄. In earlier days, folks might have produced it from carbon disulfide and chlorine, but concerns about emissions and process efficiency led to the switch. Both old and new methods create hazardous byproducts, so producers install scrubbers and advanced containment to capture and neutralize waste. Efficiency and yield depend on controlling reaction conditions and ensuring safety measures work as intended, since runaway reactions can vent poisonous gasses or other halogenated byproducts.
Chemical Reactions & Modifications
Carbon tetrachloride finds a home in organic chemistry, mainly as a solvent and reactant in halogenation reactions. It resists attack by acids and bases under moderate conditions, preserving its structure through many mild chemical processes. On contact with strong nucleophiles or under high energy, it can decompose, yielding chloride ions or less-volatile chlorinated methanes. Heating or exposure to flame may liberate highly toxic gases like phosgene or hydrogen chloride. Synthetic chemists often use it to introduce chlorinated groups or as a phasing agent during Grignard reactions, partly due to its inert behavior. Modifications to its structure, such as partial reduction or hydrolysis, lead toward simpler molecules like chloroform or dichloromethane, but most labs save these transformations for waste treatment strategies or accidental decompositions, not product synthesis.
Synonyms & Product Names
Industry jargon often shortens carbon tetrachloride to “CCl₄.” Other common names include tetrachloromethane and benzinoform. Suppliers sometimes sell under legacy trade names, especially in older technical literature, but regulatory frameworks in the European Union, North America, and East Asia encourage standardization around proper chemical identifiers. Journals and technical documents also refer to it in shorthand, reflecting its familiarity among chemists old enough to have used it more widely. Remembering these synonyms helps when reviewing older patents or product safety records, since not every document adopts the latest nomenclature or cataloging method.
Safety & Operational Standards
Anyone who has worked in labs or industrial plants keeps a sharp awareness of carbon tetrachloride’s risks. This liquid passes easily through the skin and respiratory system. Short-term exposure can bring on dizziness, nausea, or liver stress, and even mild inhalation episodes deserve immediate medical attention. Chronic exposure, especially in the days before strict oversight, caused severe liver and kidney damage, contributing to regulatory bans. Factories limit workplace concentrations through sealed systems and air monitoring, and anyone handling open containers needs gloves, goggles, and chemical fume hoods. Signs warn about its low flash point and acute toxicity, and every operation plans for spills by stocking cleanup kits and medical supplies. Training for all users covers safe storage, labeling, and emergency response, since even old, forgotten stock can still threaten health.
Application Area
Consumer and agricultural uses faded after the 1970s restrictions, but carbon tetrachloride has not disappeared from industry. Refiners rely on it as a process solvent, especially to extract valuable compounds in petrochemical streams. Some research teams turn to it when no alternate solvent matches its performance in certain spectroscopic or purification experiments. Laboratories preparing metal complexes or trying to run historic reactions still reach for CCl₄ to recreate legacy results, but alternative solvents do most of the heavy lifting today. A few specialized sectors, such as analytical chemistry or old equipment maintenance, maintain stocks under lock and key, but reporting requirements mean every molecule has to be tracked from shipment to waste.
Research & Development
Today’s research on carbon tetrachloride circles mostly around toxicity, alternatives, and waste destruction, not applications. Academics track minute quantities in air and water, using advanced chromatography and mass spectrometry to chart environmental fate. Efforts focus on photochemical and catalytic degradation: how to destroy residues safely and prevent air or groundwater contamination. Teams designing new solvents or process aids draw on lessons from CCl₄’s legacy but shift away from persistent, bioaccumulative chemicals. Historic data, stretching across thousands of peer-reviewed publications, still shape how toxicologists and epidemiologists interpret risk for other haloalkanes. Regulators fund studies to monitor legacy pollution sites, while chemical engineers tweak scrubbers and incinerators for better waste treatment. The main innovation in carbon tetrachloride work these days lies in remediation, not reinvention.
Toxicity Research
Decades of evidence -- in animal studies and unfortunate human incidents -- mark carbon tetrachloride as highly toxic. Its breakdown in the liver leads to free radicals that destroy cells and trigger fat accumulation, a precursor to cirrhosis. Acute inhalation or ingestion produces central nervous system depression, kidney failure, and sometimes death. The chemical’s long half-life in soil and ability to leach into groundwater create environmental exposure risks even long after primary use stops. Occupational studies show clusters of liver disease among workers exposed before safety standards improved, and communities near production or disposal sites continue facing elevated cancer risks. Researchers model health impacts with advanced in vitro and in vivo approaches to strengthen guidelines around air and water contamination. These findings drive tough regulations and strenuous cleanup campaigns, balancing economic activity with clear warnings shaped by past mistakes.
Future Prospects
Nobody expects carbon tetrachloride to reclaim its former place, and for good reason. Safer and more sustainable solvents continue to replace it, sparked by mounting regulatory costs and environmental liabilities. Chemical manufacturers gear toward green chemistry, working up new formulations that avoid halogenated hydrocarbons entirely. Environmental engineers focus on treating historic waste and dismantling stockpiles in obsolete facilities. Some researchers track the global fate of carbon tetrachloride—especially its slow release from old disposal sites—using satellite data and real-time environmental monitoring. For the handful of processes that still require its unique chemistry, strict containment, rapid response plans, and meticulous reporting set the standard. The story of carbon tetrachloride reminds anyone involved with chemicals that technological progress and vigilance walk hand-in-hand, especially when costs can linger for generations.
Where Carbon Tetrachloride Shows Up
Anyone who's spent time around old auto shops or heard stories from grandparents might have come across carbon tetrachloride. Its history stretches back over a century, as it played a big role in cleaning and manufacturing. It cropped up in fire extinguishers, refrigeration, and cleaning fluids. At one point, dry cleaners swore by it for getting out tough stains. Makers of refrigerants like Freon also put carbon tetrachloride to work as a raw material. On the factory floor, lab techs still use it as a solvent because it can dissolve fats, oils, and waxes that would trip up other chemicals.
Why Carbon Tetrachloride Has a Complicated Legacy
Few chemicals walk a line quite as sharp as carbon tetrachloride. There’s no getting around the fact that it cleans impressively well and serves as a handy chemical starting point. But those qualities come with a heavy toll. Breathing its fumes or working without proper protection can lead to liver damage and even failure. Nobody should handle this stuff lightly. Studies show clear links between carbon tetrachloride exposure and cancer risks. Environmental agencies like the EPA started clamping down on its widespread use after recognizing these dangers.
Its damage doesn't end in the body. Carbon tetrachloride moves through soil and can get into groundwater, threatening public water supplies. I once spoke with a water treatment operator who described the headaches of dealing with chemical contamination, tracing old industry sources back decades. Once it's in the environment, carbon tetrachloride doesn't just disappear either. It hangs around, making cleanup tough.
Modern Handling and Oversight
Chemical manufacturers don’t stop needing solvents or intermediates for their reactions, but attitudes have changed. Strict workplace rules, regular air monitoring, and the push for less harmful substitutes limit how often workers run into carbon tetrachloride these days. The United States banned its use in household products, and other countries have followed. That said, demand hasn’t disappeared—limited, careful industrial uses remain.
The people who work with dangerous chemicals wear more layers of protection than ever. Goggles, chemical suits, and specialized ventilation systems reduce the odds of a bad accident. Labs use closed systems that lock away fumes. Accidents do happen, though, and it's the communities living near older chemical plants who worry most about legacy pollution. A family friend once struggled with mysterious illnesses traced back to water supplies affected by old chemical dumps, making clear the price paid for decades of loose regulations and rushed industrial growth.
Moving Toward Safer Alternatives
Chemical safety isn’t just a matter of following rules—it's about finding smarter ways to do the same job. Researchers look for solvents with less risk for workers and the planet. Companies draw up emergency plans, set up better cleanup systems, and invest in tech that detects spills quickly. It takes real investment and listening to both workers and residents who live near manufacturing sites.
The story of carbon tetrachloride shows how the quest for convenience can backfire. It’s a reminder to weigh progress with caution, find out what goes into our daily products, and push industries to pick safety over shortcuts. Better awareness and strong oversight help protect both workers and neighbors. That’s the only path forward for chemistry that changes lives without the silent hazards.
Former Cleaning Hero Turned Health Villain
I grew up hearing stories from older family members about soaking stained shirts in home-brewed cleaning blends, some containing chemicals with long, difficult names. Carbon tetrachloride used to show up everywhere—from dry cleaning solutions to fire extinguishers. Back then, no one batted an eye about splashes and whiffs during a busy day’s work. But hidden risks don’t care about tradition or convenience.
What Science Says About Carbon Tetrachloride
Today, facts tell a sharp story. Breathing in carbon tetrachloride fumes or letting it touch your skin opens the door for trouble. Experts at the Centers for Disease Control and the Environmental Protection Agency spell it out: the stuff packs a punch to the liver and kidneys, along with other organs. Swallowing even small amounts—or inhaling fumes in stuffy rooms—brings headaches, confusion, and dizziness. Larger exposures set the stage for nausea and sudden collapse.
No mystery surrounds long-term risks, either. Persistent, low-level contact can chip away at a liver until serious disease sets in. Kidney damage follows closely. More troubling, heavy-duty studies support a link between carbon tetrachloride and several kinds of cancer in animals. It’s no leap to worry about similar risks for humans, though scientists keep looking for the final proof.
Real-World Experience and Lessons
People who used to fill and fix home fire extinguishers or work long hours in cleaning shops share real stories of sickness. Some recall weird tastes on their tongues after a busy day, others describe days of feeling groggy for no clear reason. It didn’t add up for a while, until workplace studies tied their symptoms to breathing lingering fumes and handling spills without gloves.
I keep thinking about those stories now that safer choices exist. Why hold on to harmful habits just because old hands worked that way decades ago? Health matters more than nostalgia.
Protecting Workers and Families
Some folks shake off chemical warnings, figuring all jobs carry a bit of risk. That mindset stalls progress. History teems with examples: people once shrugged at asbestos dust and lead paint, too. Real change sprang from listening to sick workers and mounting medical evidence, not corporate lobbyists or industry pride.
Nobody working around cleaning solvents, degreasers, or outdated fire extinguishers should skip goggles and gloves. Fresh ventilation in workplaces—open windows, strong fans, and up-to-date filters—should be non-negotiable. Training needs regular refreshers, geared toward recognizing symptoms of exposure before real damage starts. Inspections and rules only matter if companies actually enforce them, not hide behind paperwork and locked cabinets.
Stepping Beyond Carbon Tetrachloride
Many industries and households have already dumped this chemical for safer alternatives. That’s possible for everyone. Choosing non-toxic cleaning products for home chores, following correct disposal guidelines for old extinguishers, and asking repair shops about their solvents go a long way. A city or company that bans dangerous chemicals proves life must weigh heavier than nostalgia or the bottom line.
So, is carbon tetrachloride hazardous? Absolutely, with risk baked into every stage of its use. Listening to both science and lived experience offers a clear path: leave this one in the past.
Understanding Carbon Tetrachloride’s Hidden Dangers
Carbon tetrachloride carries a past as a cleaning solvent and fire extinguisher filler, but its toxic legacy remains a huge concern. I remember reading about how easily it can evaporate and hurt the liver or kidneys even at low concentrations. Breathing it or touching it won’t make you sick straight away, but the danger grows quietly. Reports from the Environmental Protection Agency have made it clear that long-term exposure can lead to cancer or organ failure. That history should guide every decision about where and how to keep this chemical.
Strong Containers and Smart Choices Mean Fewer Accidents
One lesson stands out to anyone handling chemicals like carbon tetrachloride — store it in tightly sealed glass or metal containers. Plastic bottles aren’t tough enough for the job because this stuff eats through many plastics and rubber seals over time. I’ve seen older storage drums in school labs leak simply because someone picked the wrong lid. Once it starts leaking, those fumes threaten everyone in the room.
Labeling can feel like a formality until you open a cabinet and find several mystery jugs. A clear, accurate label with hazard information, date received, and a contact number makes a big difference. It saves someone from a surprise years later when the original user has moved on. Double-checking secondary containment like trays or spill pallets always adds another layer of safety, especially if the main container gets knocked over.
Letting Carbon Tetrachloride Breathe Free Spells Trouble
Fumes from carbon tetrachloride are heavy and sink to the ground, collecting in low places. I learned early on that this matters in storage rooms. Even a tiny leak can turn a safe-seeming area into a danger zone, especially without good air flow. The right storage means a cool, dry, well-ventilated space that’s kept away from sunlight, heat sources, or ignition points. Direct sunlight raises pressure inside containers, pushing fumes out, and heat may spark problems with other chemicals nearby.
Open flames and reactive substances stay far away. It reacts badly with aluminum, alkali metals, and certain chemicals that might be found in an older chemical storeroom. Store carbon tetrachloride in a separate, locked cabinet. During college, one slip-up led to a spill right on an old, rusty shelf, eating through multiple items before someone caught it. Segregation isn’t just for the rulebook—it’s basic risk control.
Mitigating Risks: People, Plans, and Honest Assessment
Having a written storage protocol means less chance of shortcuts becoming habits. Training goes beyond quick videos — handling mock drills, reviewing safety data sheets, and encouraging staff to flag problems give everyone a stake in safety. Facilities benefit from regular inspections and removing outdated or damaged containers. If a facility can’t maintain proper controls or control access, it’s time to move those chemicals to a specialized facility. I’ve had to advocate for these changes a few times when corner-cutting almost led to disaster.
If a facility finds itself with carbon tetrachloride it can’t handle safely, contacting a licensed disposal service becomes urgent. Professional disposal avoids risking human health and avoids environmental fines. Respect for the chemical, ongoing learning, and honest housekeeping offer the best line of defense.
Understanding the Real Risks
Carbon tetrachloride doesn’t belong on a list of hobby chemicals. I’ve seen folks treat hazardous solvents as if gloves and a quick rinse will take care of anything. But carbon tet is a heavy hitter. At one point, people used it for dry cleaning and fire extinguishers. Then doctors started noticing more people coming in with liver and kidney problems. The clue pointed straight to this clear, sweet-smelling liquid. Carbon tet carries a well-earned reputation as a toxic chemical—and not just a little bit. So anybody working with it must give safety the same attention as the work itself.
Personal Protection: The First Line of Defense
Once you plan on using carbon tet, you pull on the right gloves. No cheap latex—nitrile or Viton stand between your skin and a potentially deadly toxin. If you don’t own a set of proper goggles, go buy some. This liquid vaporizes quickly, so safety glasses won’t cut it. The fumes sting the eyes and go right for your lungs. A full face shield never feels like an overreaction. Even on a quick job, a splash can land you in the hospital.
Respirators aren’t for show either. A standard dust mask won’t keep the vapors out of your lungs. A mask with the right cartridges, rated for organic vapors, keeps exposure down. The difference isn’t subtle. Breathing in carbon tet vapors can make you dizzy, confused, even knock you out. Long-term contact hits the liver and kidneys hardest, and the risk of cancer isn’t some distant concern—studies link the compound clearly to higher rates.
Keep It Contained
Every time I’ve visited an older lab, I spot improvised setups that tempt fate. Working with carbon tet in an open room deserves a hard no. Always do this under a working chemical fume hood. If that’s not possible, leave the job for a better-equipped space. Prolonged exposure gets you in trouble before you know it. Ventilation ranks just as high as gloves and goggles.
Storage takes planning. Carbon tet sits best in tightly sealed glass or steel containers, clearly labeled, and kept away from heat or flames—this liquid burns off toxic phosgene and hydrochloric acid if it gets hot. Never keep it near oxidizers or acetone; it reacts and kicks off dangerous gases. Spills need absorbent material and proper disposal. Never throw rags soaked in carbon tet in the trash. Follow your local hazardous waste rules. A small mess, if ignored, means chronic fumes in the air and mounting health problems.
Practical Training and Emergency Response
Even old hands get caught off guard. So refresher courses matter. Memorizing the emergency steps means you don’t freeze up. If you spill, evacuate the area, grab your chemical spill kit, and call for trained help. Don’t let anyone unprotected inside. For skin contact, you flush with running water for at least 15 minutes. Take that seriously. Inhalation victims need fresh air, and if they get sick, someone calls for medical help right away.
Culture of Respect, Not Fear
Respect for carbon tet comes from knowing its history and the problems caused by shortcuts. The science behind its dangers is settled, and nobody wants to be the example that shows why safety procedures matter. Handling this chemical calls for focus, preparation, and a willingness to say no if everything isn’t ready. Trust me—cutting corners with carbon tet doesn’t save time; it only trades safety for risk that never needed to exist.
A Chemical with a Checkered Past
Carbon tetrachloride once stacked supermarket shelves as a stain remover. Folks used it for dry cleaning at home. Decades ago, I remember my grandfather keeping a bottle in the basement, not knowing the risks. People hoped these solvents would make life easier. Now science tells a different story. This stuff is toxic. Studies link it to liver and kidney damage and even cancer. Breathing high concentrations for too long can flat-out ruin your health. That’s not speculation—it’s well-documented.
Laws Hit the Brakes
Regulation didn’t arrive overnight. In the United States, the EPA recognized the health hazards early. Carbon tetrachloride now lands on some of the strictest lists: the Clean Air Act classifies it as a hazardous air pollutant, while OSHA limits workplace exposure. European countries approached the problem from a different angle. The European Union prohibits almost all uses under REACH legislation. Companies in France, Germany, and the Netherlands stop using it for dry cleaning and fire extinguishers. Today, most applications vanished from Europe’s market.
Australia and Canada responded with their own bans. Australia phased out large-scale use, keeping its chemical industry under tough scrutiny. Canada restricts use so harshly that outside a handful of laboratories, no one sees this chemical anymore. Even developing countries joined in under the Montreal Protocol, which called for a total end to its production for most uses because of its role in burning a hole through the ozone layer.
Lingering Loopholes and Global Risks
I’ve seen reports where unintended production still pops up—industrial chemistry isn’t perfect. When you mix chemicals at massive scale, small amounts can slip through as byproducts. Carbon tetrachloride keeps showing up in some manufacturing plants in Asia and South America. Lax enforcement in these places makes the chemical tough to stamp out. Smugglers sometimes move it across borders, especially where other environmental laws go ignored.
Cheap raw materials and old habits die hard. In countries without real oversight, the old patterns persist, and the health risks follow. People working on factory floors keep running into exposure—often without the protective gear so common in Europe or North America. Symptoms crop up years later: fatigue, liver pain, even hospital visits without a clear diagnosis.
Better Solutions for a Safer World
Tighter regulation matters, but it’s not enough. Public education must push just as hard. People need to know what’s in their homes and workplaces. When my cousin in Southeast Asia asked why he felt sick handling old solvents, knowledge became the turning point. Powerful regulations must pair with outreach that speaks to workers and families directly.
Technology plays a part. Cleaning formulas dropped carbon tetrachloride after safer alternatives arrived. Industry leaders turned to water-based solvents and new synthetic blends that don’t poison workers or the planet. Incentives for companies to upgrade equipment go a long way in low-income communities. Governments can support these efforts with funding and clear legal guidelines—just enforcing what’s on paper isn’t enough.
Eliminating carbon tetrachloride is possible. Countries with strict controls show that life goes on without it, and nobody needs to gamble with their health for the sake of clean clothes or cheap chemical processes. The fight requires courage, information, and a global approach. No one should pay the price for shortcuts when better options exist for everyone.