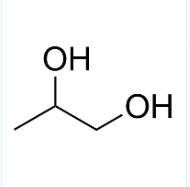
1.What is Propylene Glycol?
The scientific name of Propylene Glycol is 1,2-propanediol. There is a chiral carbon atom in the
molecule. The racemate is a viscous liquid that is easily hygroscopic and has a slight spicy taste.
Missoluble in water, acetone, ethyl acetate, and chloroform, soluble in ether. Soluble in many
essential oils, but not miscible with petroleum ether, paraffin, and fats. Stable to heat and light,
more stable at low temperatures. Propylene Glycol can be oxidized to acetaldehyde, lactic acid,
pyruvate, and acetic acid at high temperatures.
2.What is Propylene Glycol used for?
Used for producing unsaturated resins, polyether polyols, epoxy resins, etc;
Used for organic synthesis, solvents, dehydrating agents, gas chromatography stationary liquids,
synthesis of emulsifiers, demulsifiers, etc;
Chemical intermediate acting on the pesticide fungicide phenyl ether metronidazole;
Used in the glass paper, plasticizers, and pharmaceutical industry;
The aqueous solution of Propylene Glycol is an effective antifreeze;
This product can be used as a carrier for human pharmaceutical drugs, as well as a granule drug
agent, moisturizer, softener, and solvent;
It can also be used as a solvent in the field of veterinary medicine and pesticide;
It can be used in tobacco industry as tobacco essence, tobacco humectant and preservative;
It can be widely used as a moisturizer in the daily chemical industry.
3.Is Propylene Glycol safe?
If Propylene Glycol can be used correctly, it generally does not pose a threat to physical
health.
Description of first aid measures:
(1).In all cases of doubt, or when symptoms persist, seek medical attention.
(2).In case ofinhalation:
Not expected to be an inhalation hazard under anticipated conditions ofnormal use ofthis
material-Avoid inhalation of hot vapors or extremely high concentrations of aerosols
-Remove to fresh air.
-Consult a physicianifnecessary
Skin contact:
-Wash skin with plenty of water
(3). In case of skin contact:
Wash skin with plenty of water
(4). In case of eyes contact:
Flush eyes with water thoroughly and continuously for 15 minutes.
4.How to securely store?
Store in a cool and ventilated warehouse, with a temperature not exceeding 37
℃ and a relative humidity controlled below 60%.
It should be stored separately from oxidants, reducing agents, acids, etc. It is important to avoid
mixing and keep the container sealed.
Stay away from sparks and heat sources. Prohibit the use of mechanical equipment and tools that are
prone to generating sparks.
The storage area should be equipped with emergency response equipment for leaks. When stacking and
storing, the bottom layer should be placed with a cushion layer, and the stacking height should not
exceed 2 meters.
5.How is Propylene Glycol made?
1.The direct hydration method of epoxypropane is a pressurized non catalytic hydrolysis method. It
is obtained by direct hydration of epoxy propane and water at 150-160 ℃ and 0.78-0.98 MPa pressure.
The reaction product is evaporated and distilled to obtain the final product. 57-55-6
preparation
2. The indirect hydration method of epichlorohydrin is obtained by the indirect hydration of
epichlorohydrin and water using sulfuric acid as a catalyst.
3. Direct catalytic oxidation of propylene.
4. Using 1,2-dichloropropane as the raw material, this method has two process routes: first,
dichloropropane is directly hydrolyzed into Propylene Glycol in weak alkaline aqueous solution; The
second is that dichloropropane reacts with carboxylates to form esters, which are then hydrolyzed
into Propylene Glycol.
(1)The direct hydrolysis process involves adding 1,2-dichloropropane, water, sodium bicarbonate, and
hexadecyltributylphosphonium bromide to a reactor. The reaction is carried out at 100 ° C and CO2
partial pressure of 1.0 MPa for 18 hours to obtain 80% Propylene Glycol. Control the feeding speed
of dichloropropane, that is, the feeding speed is fast at high temperatures and slow at low
temperatures. Example: Add 60g of calcium carbonate and 150g of water to a 300ml high-pressure
vessel, stir and heat up to 230 ℃, and continuously add dichloropropane at a rate of 0.03g/(min ·
100gH2O) for 11.5 hours; Continue stirring at this temperature for 30 minutes, then rapidly cool to
room temperature. The yield of Propylene Glycol is about 95%. By controlling the temperature between
130-300 ℃ and changing the feeding rate of dichloropropane accordingly, the yield of Propylene
Glycol can reach over 95%.
(2) The two-step hydrolysis process first reacts the raw materials in a kettle reactor. After
reaching a certain conversion rate of dichloropropane, it is fed into a piston flow reactor for
further reaction, and finally hydrolyzed into Propylene Glycol.