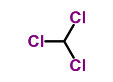
What is Chloroform?
This product is a colorless transparent liquid with strong refraction. It has a special sweet smell.
Not easy to burn. When exposed to sunlight or oxidized in the air, it will gradually decompose and
produce phosgene (carbonyl chloride). Therefore, 1% ethanol is usually added as a stabilizer. It can
be miscible with ethanol, ether, benzene, petroleum ether, carbon tetrachloride, carbon disulfide,
and oils. 1 mL of this product can be dissolved in about 200 mL of water (25°C). Generally, it will
not burn, but it can still burn when exposed to open flame and high temperature for a long time. It
will decompose under excessive water, light and high temperature, and produce highly toxic and
corrosive phosgene and hydrogen chloride. A strong base like lye and potassium hydroxide can break
down chloroform into chlorate and formate. Under the action of strong alkali and water, it can form
explosives. When in contact with water at high temperature, it is corrosive, corrodes iron and other
metals, corrodes plastics and rubber.
The fire-fighting measures?
Hazardous characteristics: It can produce highly toxic phosgene when it comes into contact with open
flames or hot objects. Under the action of air, moisture and light, the acidity increases, so it is
strongly corrosive to metals.
Hazardous Combustion Products: Hydrogen Chloride, Phosgene.
Fire extinguishing method: Firefighters must wear filter gas masks (full face masks) or isolated
respirators, and wear full-body fireproof and anti-virus clothing, and put out the fire in the
upwind direction.
Extinguishing media: mist water, carbon dioxide, sand.